1 Abbott first made the test available as part of the US. Researchers at the University of Washington School of Medicine found the test manufactured by Abbott Laboratories had a specificity rate of 999 and a sensitivity rate.

Abbott Launches Covid 19 Antibody Test Abbott Newsroom
Im very excited to be able to share with you what I have learned over the past many years of study and hands-on practice with thousands of patients.

Abbott laboratories covid 19 antibody test accuracy. An antibody blood test for covid-19 which the manufacturer Abbott claims is 99 accurate has been certified for use by the European Union. Abbott Labs Covid 19 Antibody Test Accuracy. New information that I find in the course of.
Abbott and DiaSorin are among the latest companies to receive emergency use authorizations from the FDA for coronavirus antibody testsThe agency posted new designations for those tests and ones from Ortho Clinical Diagnostics and Autobio Diagnostics over the weekend adding to prior EUAs awarded to Cellex Chembio Diagnostic System Mount. Rapid antigen tests such as the Abbott BinaxNOW COVID-19 Ag Card BinaxNOW offer results more rapidly approximately 1530 minutes and at a lower cost than do highly sensitive nucleic acid amplification tests NAATs 1Despite a lower sensitivity to detect infection rapid antigen tests can be an important tool for screening because of their quick. Ratings 78 December 2021 News.
Abbott on Monday announced the FDA granted emergency use authorization for a test meant to detect antibodies to the coronavirus that runs on its Alinity i immunoassay platform. Abbott Labs Covid Antibody Test Accuracy. Our Rapid COVID-19 Tests Our BinaxNOW test is the size of a credit card and requires no specialized instrumentation.
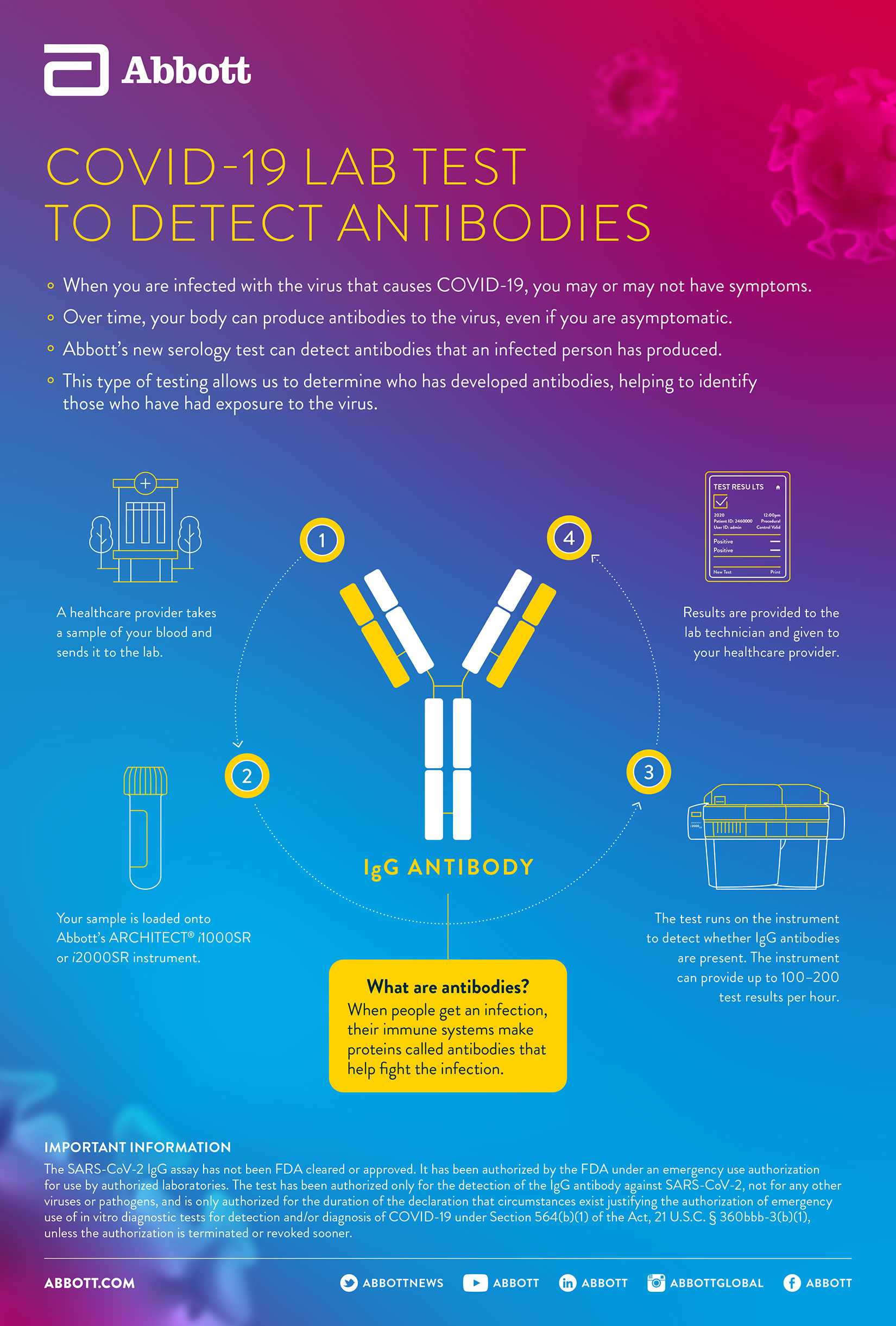
Find where Abbotts COVID-19 antibody testing is available in the US. The test will identify if a person has had the novel COVID-19 and will further understanding of the virus including how long antibodies stay in the body and if they provide immunity. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 CLIA 42 USC.
Our antibody tests for ARCHITECT and Alinity i systems have received both Emergency Use Authorization in the US. The SARS-CoV-2 IgG assay has not been FDA cleared or approved. Highly accurate antibody increasingly available but doctors warn against misinformation.
A new antibody test is highly accurate at determining whether people have been infected with the novel coronavirus according to a study published on Friday in The Journal of Clinical Microbiology. And CE Mark in Europe. Late last month the companys SARS-CoV-2.
It will be available at major US. Updated at 1116 pm. Food drug and mass merchandiser retailers in.
Abbott Labs Covid Antibody Test Accuracy - Safely Save Your money. The Food and Drug Administration is cautioning the public about the reliability of a widely used rapid test for the. The testwhich has received its CE mark meaning that it complies with EU safety rulesdetects the antibody IgG to identify whether a person has had covid-19.
Abbott labs coronavirus antibody test achieves high performance in study. Ratings 78 December 2021 News. The EUA marks the fourth for a test from Abbott addressing COVID-19 and its second focused on the IgG antibody.
Researchers at the University of Washington School of Medicine found the test manufactured by Abbott Laboratories had a specificity rate of 999 and a sensitivity rate. A new antibody test is highly accurate at determining whether people have been infected with the novel coronavirus according to a study published on Friday in The Journal of Clinical Microbiology. Abbott has announced the immediate availability of its lab-based antibody test for COVID-19 after it was granted a CE Mark.
The Abbott test also tells you that the antibodies the test detected are antibodies to the COVID-19 virus 9963 of the time. COVID-19 serology tests from Roche and Abbott were reported by the manufacturers and government last week as being 100 accurate and as game-changers in the identification of past infection with COVID-19. According to Abbott it has demonstrated specificity and.
This test is authorized for use with direct anterior nasal nares swab samples from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 36 hours between tests. Abbott Laboratories said an ongoing study showed that its ID NOW Covid-19 test had a high rate of accuracy as the company attempts to counter a claim by outside doctors that the test may return too many false negatives. Abbott was not the first company to get emergency use authorization for a point-of-care coronavirus test Cepheid claimed that milestone.
Partial data from the company-funded study showed that it accurately detected the virus 947 of the time and correctly gave negative results. The BinaxNOW COVID-19 Ag Card is available in the US. The test has been authorized only for the detection of the IgM antibody against SARS-CoV-2 not for any other viruses or pathogens and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection andor diagnosis of COVID-19 under Section 564b1 of the Act.
Im very excited to be able to share with you what I have learned over the past many years of study and hands-on practice with thousands of patients. The new antibody test is to be used on Abbotts ARCHITECT i1000SR and i2000SR laboratory instruments which can run up to 100-200 tests an hour. The tests have been authorized only for the detection of proteins from SARS-CoV-2 not for any other viruses or pathogens and are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection andor diagnosis of COVID-19 under Section 564b1 of the Federal Food Drug and.
These claims were based on studies undertaken by Public Health England PHE but the release of these statements preceded any official study reports being. Abbott Labs Covid 19 Antibody Test Accuracy - Safely Save Your money. Study suggests Abbott Covid-19 antibody test highly likely to give correct results.
Similarly Abbotts AdviseDx SARS-CoV-2 IgM antibody test has a 9956 specificity and 95 sensitivity for patients tested 15 days after symptoms started. Abbotts rapid tests are among the most widely-used in the US with more than 200 million of our BinaxNOW and ID NOW rapid tests used in urgent care clinics doctors offices pharmacies nursing homes and schools since April 2020. A new antibody test is highly accurate at determining whether people have been infected with the novel coronavirus according to a study published on Friday in The Journal of Clinical Microbiology.
New information that I find in the course of my. Food and Drug Administrations notification without an Emergency Use Authorization EUA pathway that was outlined for COVID-19 diagnostic tests during the. This is called the specificity of the test.
Abbott said that the test had demonstrated specificity and. But the potential for Abbotts test to detect positive cases in five minutes and negative results in 13 minutes as well as the companys claim its ID Now machines were the most widely available molecular POC testing. 846 for detecting covid-19 infections 985 for correctly identifying covid-19 negatives This is the at-home version of the fast.
Previous studies of BinaxNOW compared with rRT-PCR have demonstrated a high negative percent.

Covid 19 Antibody Tests Aren T A Magic Bullet To Escape Lockdown
Abbott 99 Effective Antibody Test Gets Eu Green Light After Us Launch

Fda Cautions About Accuracy Of Widely Used Abbott Coronavirus Test Coronavirus Updates Npr
Abbott Launches Covid 19 Antibody Test Abbott Newsroom

Fda Issues Warning On Accuracy Of Abbott S Rapid Coronavirus Test After Study Finds False Negatives
Komentar
Posting Komentar